The interim approach proposed a “status quo” to pricing until Guidelines could be established relating to the final regulations. As we previously reported, the final regulations included the new “basket” of foreign reference countries but repealed the provisions relating to: (i) additional price regulatory factors and related reporting requirements, and (ii) the collection of price and revenue information net of third-party rebates. Neither the current Guidelines (last updated in February 2017) nor the 2020 Guidelines address the final regulations.
The Board had invited comments on the proposed interim approach to pricing and has published the 37 submissions received.
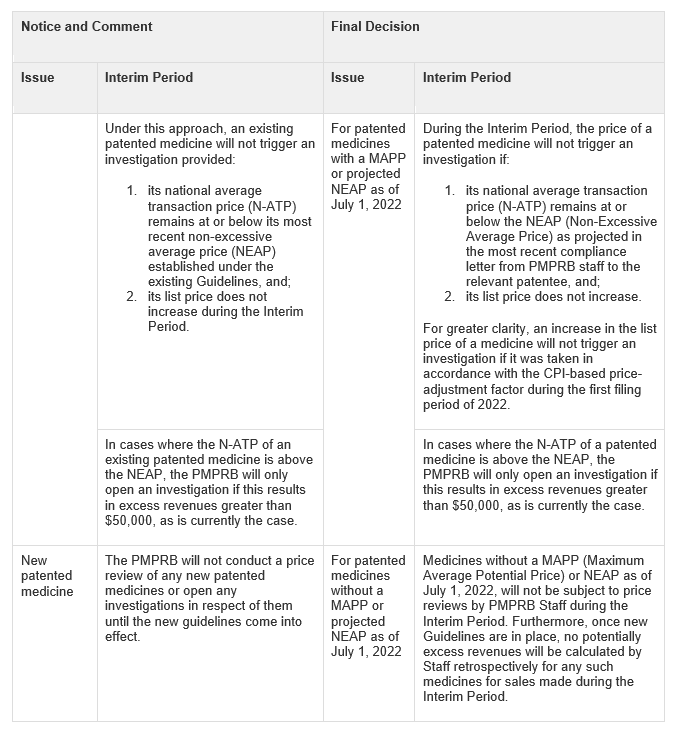
A final decision on the interim approach was announced on August 18, 2022. The Board has largely maintained its proposed approach, noting the following distinctions between the original proposal and the final decision:
The announcement also includes FAQs addressing the questions:
- How can patentees know what the NEAP is that will apply to the price of a medicine during the Interim Period?
- Will the NEAPs continue to apply to the price of medicines if new Guidelines are not in place by the end of 2022?
- How will the PMPRB address the value of free and compassionate goods during the Interim Period?
- Will HDAP meetings continue on their current timelines for new (and future) medicines?
Guideline consultations are expected to begin in September of this year, and the Board has committed to completing the consultation process and issuing new Guidelines before January 1, 2023.
In the meantime, should you have any questions, please do not hesitate to contact a member of the Life Sciences Regulatory & Compliance Group.
The preceding is intended as a timely update on Canadian intellectual property and technology law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
Related Publications & Articles
-
-
New PMPRB Chairperson and PMPRB releases January 2026 NEWSletter
On January 29, 2026, the Minister of Health announced Anie Perrault, former Vice-Chairperson, as Chairperson of the Patented Medicine Prices Review Board (PMPRB) until August 9, 2028.Read More -
Canada’s Drug Agency announces consultation on streamlined review process and new reconsideration/resubmission procedures
On January 29, 2026, Canada’s Drug Agency (CDA) published Improvements to the Drug Reimbursement Review Process, which includes a section for consultation on proposed changes to the reimbursement re...Read More
