Since 2018, Health Canada has undertaken an initiative to adapt its regulatory approach to better support digital health technologies, specifically medical devices. Key focus areas include artificial intelligence, software as a medical device, cybersecurity, medical device interoperability, wireless medical devices, mobile medical apps and telemedicine. To meet this goal, Health Canada established the Digital Health Division under the Medical Devices Bureau and has been increasing its efforts to build in-house expertise.
On October 27, 2021, Health Canada, the US Food and Drug Administration (FDA), and the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) jointly published the Good Machine Learning Practice for Medical Device Development: Guiding Principles. The document consists of 10 guiding principles to help promote safe, effective, and high-quality use of artificial intelligence and machine learning (AI/ML) in medical devices. Artificial intelligence is a field that combines computer science with data to enable machines to mimic human intelligence when solving problems. Machine learning is a sub-field of artificial intelligence where algorithms are designed to learn and improve a machine through experience (i.e., new data). These algorithms can be “locked”, so their function does not change, or “adaptive”, meaning their behaviour can change over time.
AI/ML medical devices have the potential to revolutionize the healthcare sector, as they continuously learn from and improve on their performance using real-world data after deployment in the market. However, such medical devices are difficult to regulate, since Health Canada requires a mechanism to ensure safety and efficacy as the medical devices “adapt” over time.
The current regulatory approach used by Health Canada for approving medical devices is not suited for the iterative and data-driven nature of AI development. Health Canada faces a variety of challenges developing an appropriate framework for AI/ML medical devices, including:
- fostering innovation without sacrificing safety and effectiveness;
- establishing manufacturer requirements for pre-market authorization;
- ensuring data sets used during development are reliable and representative;
- setting ideal performance metrics to make proper assessments on algorithm performance;
- ensuring AI integration is done appropriately without unintended consequences;
- developing a framework for post-market regulation; and
- clarifying rules on accountability if mistakes are made by software.
The Guiding Principles mark a continuing effort by Health Canada to adjust its approach to better regulate and facilitate market access of adaptive AI/ML medical devices.
The ten guiding principles are:
- Leveraging multi-disciplinary expertise throughout the total product life cycle to ensure safe and effective AI/ML medical devices.
- Implementing good software engineering and security practices, including good data quality assurance, data management, cybersecurity, and methodical risk management and design processes.
- Using clinical study participants and data sets that are representative of the intended patient population (factors include age, gender, sex, race, and ethnicity).
- Using training data sets that are independent of test data sets. Training data sets refer to the data used to train a medical device, whereas testing data sets refer to the data used to test a medical device after it has been trained to measure performance, accuracy, and efficiency.
- Developing reference data sets that are based upon best available methods to ensure that clinically relevant and well-characterized data are collected and limitations of the reference data sets are understood.
- Tailoring the model design to the available data and ensuring the model design reflects the intended use of the device.
- If humans are involved in the model, paying particular focus on the human-AI performance rather than AI performance in isolation.
- Developing and using sound testing plans that demonstrate device performance during clinically relevant conditions.
- Making available and providing clear and contextually relevant information to users, including healthcare providers and patients.
- Monitoring the performance and managing retraining risks after models are deployed.
Although there are active AI/ML medical devices approved by Health Canada in the market today, these devices use locked, rather than adaptive, AI algorithms. The table below illustrates some of the AI/ML medical devices that have been approved by Health Canada.
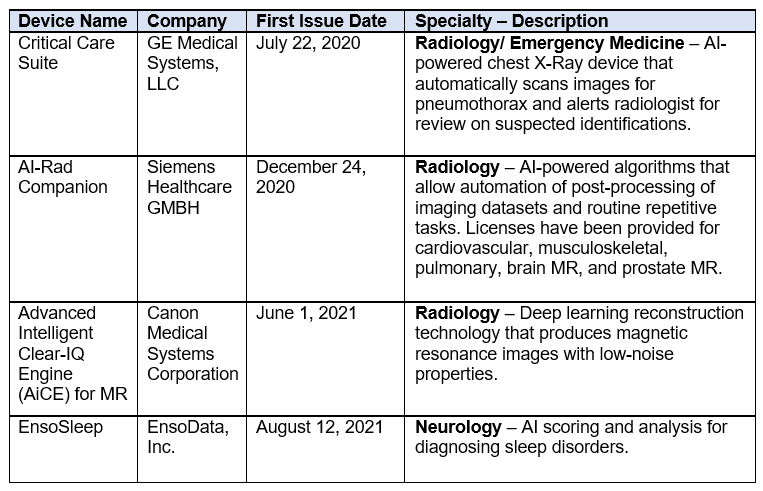
These Guiding Principles are the latest example of Health Canada taking steps to modernize its regulatory approach to dealing with emerging technologies like AI/ML medical devices. These steps are critical for fostering AI innovation in the healthcare sector without sacrificing safety and effectiveness of the medical devices for its users. Although challenges remain, it is encouraging to see Health Canada taking a systematic approach and collaborating with its international counterparts.
Should you have any questions, please do not hesitate to contact a member of the Life Sciences Regulatory & Compliance group.
The preceding is intended as a timely update on Canadian intellectual property and technology law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
Related Publications & Articles
-
Top five 2025 trends in Canadian copyright law
2025 saw incremental developments in Canadian copyright matters anticipated to set the foundation for potentially major changes in the coming years in AI and accessible remedies for infringement and h...Read More -
Patenting AI: Shift the focus to do it better
Patent protection is pursued for all types of technologies. Why should anything be different just because the technology is based on artificial intelligence (AI)? Nothing is different when reduced to...Read More -
Update on proposed Ministerial Reliance Order
As we reported last month, the Minister of Health launched a consultation on a proposed Ministerial Reliance Order that would permit Health Canada to complete the examination of certain parts of a dru...Read More
