Update: See article here describing the June 2020 revised draft Guidelines and here describing the final October 2020 Guidelines. On November 23, Innovative Medicines Canada and a number of research-based pharmaceutical companies commenced an application for judicial review of the final Guidelines. The definition of “Gap medicines” and compliance timelines were updated following a consultation (see article here), as were compliance timelines for Grandfathered and Gap medicines (see article here). A consultation is underway regarding a further update to the definition of Gap medicine, and other changes (see article here).
Yesterday, the Patented Medicines Prices Review Board (“PMPRB”) released draft new Guidelines for consultation, together with a backgrounder (see also news release here). The new Guidelines are intended to operationalize the amended Patented Medicines Regulations, which come into force on July 1, 2020. The deadline for providing written submissions is January 20, 2020.
Noteworthy changes to the Regulations include:
For drugs that received marketing approval (i.e., that have been assigned a Drug Identification Number, or “DIN”) before August 21, 2019 (“grandfathered medicines”) or on or after August 21, 2019 (together, “all medicines”),
- An updated list of reference countries (revised Schedule), notably excluding the United States and Switzerland (the “PMPRB11”);
- Reporting of price and revenue net of adjustments including third party price rebates (amended s. 4(4)); and
- Reduced reporting requirements for drugs considered at low risk of excessive pricing (new s. 3(3.1), amended s. 4(3), and new s. 4.3(1)).
In addition, for drugs that received a DIN on or after August 21, 2019 only (“non-grandfathered medicines”),
- Three new price regulatory factors (new s. 4.4): pharmacoeconomic value, market size, and gross domestic product (GDP) in Canada and GDP per capita in Canada; and
- Added reporting requirements relating to the new price regulatory factors (new ss. 4.1 and 4.2).
For a more detailed discussion of the amendments, see our previous articles here and here, and our summary of pending Court challenges to the amended Regulations here. The Federal Court proceeding has been scheduled to be heard in April 2020.
A brief summary of the proposed price review process follows.
i. Price review process for non-grandfathered medicines
For medicines assigned a DIN on or after August 21, 2019, the draft Guidelines provide the following schematic showing the circumstances under which a list price or average transaction price (ATP) may be subject to investigation (shown with added yellow highlighting).
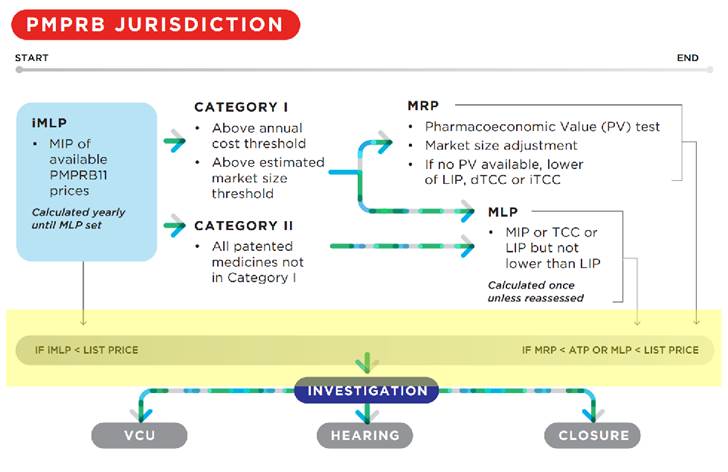
The draft Guidelines outline a two-step process for the price review of non-grandfathered medicines.
In the first step, an interim maximum list price (iMLP) will be set based on the median international list price (MIP) for the PMPRB11 countries. The ex-factory price of the medicine in Canada cannot exceed the iMLP during the interim period.
In the second step, following the defined interim period, the iMLP will be replaced by the maximum list price (MLP). The MLP will be the lower of the MIP or the result of the median domestic Therapeutic Class Comparison Test (dTCC) (see Appendix A) but is subject to a floor set by the lowest international price (LIP).
Medicines classified as Category I will be subject to an additional pricing constraint – the maximum rebated price (MRP), which takes into account the pharmacoeconomic value and market size for the medicine. A medicine will be classified as Category I if either 12 months of treatment costs greater than 50% of GDP per capita (currently about $28K) or if the estimated or actual market size exceeds $25M annually. Category 2 covers all medicines not in Category I and includes line extensions of grandfathered medicines receiving a DIN on or after August 21, 2019 where the DIN does not relate to a new indication.
The MRP is calculated as follows:
- The pharmacoeconomic price (PEP) is calculated by determining the price at which the medicine’s incremental cost-effectiveness ratio (ICER) would be equivalent to the pharmacoeconomic value threshold (PVT) of $60,000 in cost per quality-adjusted life years (QALYs) (see Appendix C);
- The PEP may be adjusted for market size if, priced at the MRP set by the PEP, annual revenues would be > $25 million (see Appendix D);
- For drugs for rare diseases (prevalence across all indications < 1 in 2000), the MRP will initially be calculated by applying an increase of 50% to the PEP, but will be adjusted for market size if, priced at the MRP set by the PEP, annual revenues would be > $12.5 million (see Appendix D); and
- If the above results in an MRP > MLP, the MRP will be set at the MLP.
ii. Price review process for grandfathered medicines
Medicines assigned a DIN before August 21, 2019 are subject to an MLP, but not an MRP. The MLP for such medicines will be the lower of (i) the MIP for the PMPRB11 countries for which the patentee has provided information, or (ii) the medicine’s price ceiling under the February 2017 version of the Guidelines.
Reassessment
The draft Guidelines also provide a number of circumstances in which categories (i.e. whether a medicine falls into Category I or Category II) or price ceilings may be reassessed. For example, for non-grandfathered patented medicines, a reassessment may be conducted if the medicine is approved for a new indication or its cost-utility analysis is updated. Also by way of example, a Category II medicine receiving a new indication may be re-categorized to Category I if it meets the Category I screening criteria.
Conclusions
The above provides only an overview of the proposed process, the details of which are complex and should be reviewed in the draft Guidelines themselves. In addition to considering written submissions, the PMPRB will also hold a policy forum and strike working groups to discuss the draft Guidelines.
The preceding is intended as a timely update on Canadian intellectual property and technology law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
Related Publications & Articles
-
Update on biosimilars in Canada – March 2024
In this article, we provide a further update on developments regarding biosimilars in Canada (approvals, pending submissions, litigation, regulatory, and market access).Read More -
Minister of Health proposes legislation for first phase of national universal pharmacare
On February 29, 2024, the Minister of Health announced the introduction of Bill C-64, An Act respecting pharmacare (Pharmacare Act).Read More -
PMPRB releases 2022 Annual Report and March 2024 NEWSletter
On February 16, 2024, the Minister of Health tabled the 2022 Annual Report of the Patented Medicine Prices Review Board (PMPRB).Read More
